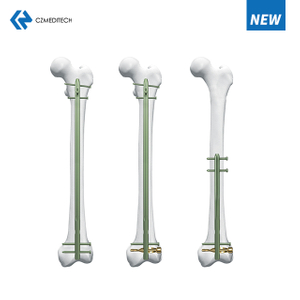
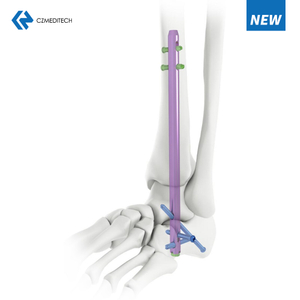
Suprapatellar Approach Tibial Nail: A Comprehensive Guide
Fractures of the tibia are common injuries that often require surgical intervention. One of the most popular surgical methods is the use of intramedullary nails. The suprapatellar approach tibial nail is a technique that has gained popularity in recent years due to its several advantages. In this article, we will discuss the suprapatellar approach tibial nail in detail, including its advantages, indications, surgical technique, post-operative management, and potential complications.
Table of Contents
Introduction
Anatomy of the Tibia
Indications for Suprapatellar Approach Tibial Nail
Advantages of Suprapatellar Approach Tibial Nail
Pre-operative Preparation
Surgical Technique for Suprapatellar Approach Tibial Nail
Post-operative Management
Potential Complications
Comparison with Other Techniques
Conclusion
FAQs
1. Introduction
The tibia is one of the most commonly fractured long bones in the body. Fractures of the tibia often require surgical intervention due to the high risk of malunion and non-union. Intramedullary nails have become the gold standard for treating tibial fractures due to their many advantages, including improved stability and faster healing times.
The suprapatellar approach tibial nail is a technique that has gained popularity in recent years due to its several advantages over other techniques. This article aims to provide a comprehensive guide to the suprapatellar approach tibial nail.
2. Anatomy of the Tibia
Before discussing the suprapatellar approach tibial nail, it is essential to understand the anatomy of the tibia. The tibia is the larger of the two long bones in the lower leg and bears most of the body's weight. The proximal end of the tibia articulates with the femur to form the knee joint, while the distal end articulates with the fibula and talus to form the ankle joint.
The tibia has an intramedullary canal that runs along its length. The canal is wider at the proximal end and narrows towards the distal end. This canal is where the intramedullary nail is inserted.
3. Indications for Suprapatellar Approach Tibial Nail
The suprapatellar approach tibial nail is indicated for the treatment of various tibial fractures, including:
Distal third tibial fractures
Proximal tibial fractures
Tibial shaft fractures
Oblique fractures
Spiral fractures
Comminuted fractures
Fractures with a significant cortical defect
4. Advantages of Suprapatellar Approach Tibial Nail
The suprapatellar approach tibial nail offers several advantages over other techniques, including:
Improved fracture reduction: The suprapatellar approach allows for better visualization of the fracture site, which leads to improved reduction of the fracture.
Reduced blood loss: The suprapatellar approach involves less soft tissue dissection, leading to reduced blood loss during the surgery.
Reduced risk of infection: The suprapatellar approach reduces the risk of infection by avoiding the knee joint, which is a potential source of infection.
Reduced risk of patellar tendon injury: The suprapatellar approach avoids the patellar tendon, reducing the risk of injury to this important structure.
Faster recovery: Patients who undergo suprapatellar approach tibial nail surgery tend to recover faster and have shorter hospital stays compared to those who undergo other techniques.
5. Pre-operative Preparation
Before undergoing suprapatellar approach tibial nail surgery, patients will typically undergo several pre-operative preparations. This will include a thorough medical history, physical examination, and imaging studies such as X-rays, CT scans, or MRI scans to evaluate the extent and location of the fracture.
Patients may also undergo pre-operative blood tests and other laboratory studies to assess their overall health and identify any pre-existing medical conditions that could affect their surgery and recovery.
It is important for patients to inform their surgeon of any medications they are taking, including over-the-counter medications and supplements, as some medications may need to be discontinued prior to surgery due to the risk of bleeding or other complications.
Patients may also be advised to quit smoking and avoid alcohol in the weeks leading up to surgery, as these substances can interfere with the healing process and increase the risk of complications.
6. Surgical Technique for Suprapatellar Approach Tibial Nail
The suprapatellar approach tibial nail surgery is typically performed under general anesthesia and may take several hours to complete. The surgical technique involves the following steps:
The patient is positioned on the operating table in a supine position, with the affected leg elevated and supported by a leg holder.
A small incision is made in the skin just above the patella, and a guide wire is inserted through the skin and into the intramedullary canal of the tibia.
A reamer is used to prepare the canal for the insertion of the nail.
The nail is then inserted through the incision and guided into the canal using a fluoroscope.
Once the nail is in place, locking screws are inserted through the nail and into the bone to secure it in place.
The incision is then closed, and the leg is immobilized using a cast or brace.
7. Post-operative Management
Following suprapatellar approach tibial nail surgery, patients will typically spend several days in the hospital for monitoring and pain management. They will be advised to keep the affected leg elevated and avoid putting weight on it for several weeks.
Patients will also be given exercises to perform to help strengthen the muscles around the knee and prevent stiffness. Physical therapy may also be recommended to help patients regain full range of motion and strength in the affected leg.
Patients will be given pain medications and antibiotics as needed to manage pain and prevent infection. Follow-up appointments will be scheduled to monitor the healing process and assess for any complications.
8. Potential Complications
As with any surgery, there are potential risks and complications associated with suprapatellar approach tibial nail surgery. These may include:
It is important for patients to discuss these risks with their surgeon and follow all pre- and post-operative instructions to minimize the risk of complications.
9. Comparison with Other Techniques
The suprapatellar approach tibial nail is one of several techniques used to treat tibial fractures. Other techniques include the infrapatellar approach tibial nail, the retrograde tibial nail, and the plate and screw fixation.
While each technique has its advantages and disadvantages, the suprapatellar approach tibial nail offers several unique advantages, including improved fracture reduction, reduced blood loss, and a reduced risk of infection and patellar tendon injury.
10. Conclusion
The suprapatellar approach tibial nail is a popular surgical technique for treating tibial fractures. It offers several advantages over other techniques, including improved fracture reduction, reduced blood loss, and a reduced risk of infection and patellar tendon injury.
However, as with any surgery, there are potential risks and complications, and it is important for patients to carefully consider their options and discuss them with their surgeon to make an informed decision.
11. FAQs
How long does the suprapatellar approach tibial nail surgery take?
The surgery typically takes several hours to complete.
How long does it take to recover from suprapatellar approach tibial nail surgery?
Recovery time can vary depending on the extent of the fracture and the individual patient's healing ability, but it typically takes several months for the bone to fully heal.
What is the success rate of suprapatellar approach tibial nail surgery?
The success rate of the surgery is generally high, but it can vary depending on the individual patient's circumstances and the extent of the fracture.
Will I need physical therapy after suprapatellar approach tibial nail surgery?
Physical therapy may be recommended to help you regain full range of motion and strength in the affected leg.
Are there any non-surgical options for treating tibial fractures?
In some cases, non-surgical options such as casting or bracing may be used to treat tibial fractures, but this will depend on the individual patient's circumstances.
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu