Gamma Intramedullary Nail – Product Overview
Video of Gamma Nail
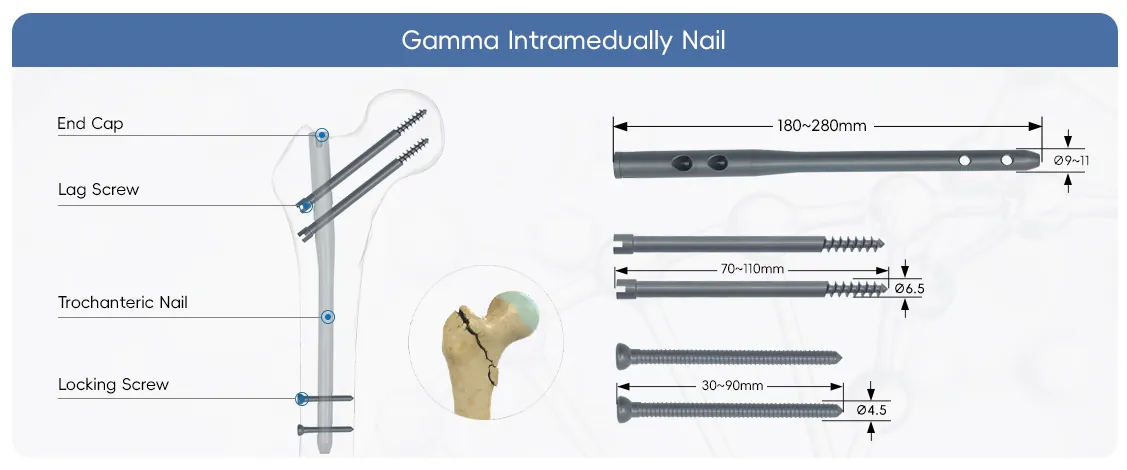
Details of Gamma Nail
The Main Nail is the core component of the Gamma Nail system, an intramedullary device engineered for the stabilization of proximal femoral fractures, particularly intertrochanteric and subtrochanteric fractures.
This is a larger diameter, solid core locking screw designed to provide maximum pull-out strength and stability in situations requiring enhanced fixation.
3.5mm locking screw is typically used for proximal locking into the femoral neck and head, often functioning as an anti-rotation screw alongside the larger lag screw.4.5mm locking screw used in various locking points of the Gamma Nail system.
The instrument set for the Gamma intramedullary nail is a comprehensive set of specially - designed tools used to ensure the safe and accurate implantation of the nail and its accessory screws.
![DFN Distal Femur Intramedullary Nail (Spiral Blade Screw Type)]()
Actual Picture of Gamma Intramedullary Nail
![DFN Distal Femur Intramedullary Nail (Spiral Blade Screw Type)]()
Clinical Advantages of Gamma Intramedullary Nail
![DFN Distal Femur Intramedullary Nail (Spiral Blade Screw Type)]()
Blog of Gamma Intramedullary Nail
Gamma Intramedullary Nail: A Comprehensive Overview
When it comes to orthopedic surgery, one of the most common procedures is the fixation of bone fractures with intramedullary nails. Among these, the gamma intramedullary nail is a popular choice due to its various advantages. In this article, we will discuss the design, indications, techniques, complications, and outcomes associated with the use of gamma intramedullary nail.
Introduction
The gamma intramedullary nail is a type of intramedullary fixation device used for the treatment of long bone fractures. It was first introduced by the AO Foundation in the 1980s and has since become a popular choice for the management of fractures in the femur, tibia, and humerus. The gamma nail is designed to provide stable fixation while preserving the biology of the fracture site and allowing for early weight-bearing.
Anatomy and Design
The gamma nail is a titanium alloy rod that is inserted into the intramedullary canal of the bone. The rod has a curved shape, which allows it to follow the natural contour of the bone. The proximal end of the nail has a flared shape, which provides rotational stability and prevents migration of the nail. The distal end of the nail has a screw thread, which engages with the cancellous bone and provides axial stability.
Indications
The gamma nail is indicated for the treatment of long bone fractures, particularly those in the femur, tibia, and humerus. It is most commonly used for fractures that are located in the middle or distal third of the bone. The gamma nail is also indicated for the treatment of fractures that are unstable or displaced, as well as for fractures that are comminuted or have a butterfly fragment.
Surgical Technique
The surgical technique for the insertion of a gamma nail involves the use of a specialized instrument set. The procedure is typically performed under general or regional anesthesia. After preparing the patient and the surgical site, a guide wire is inserted into the intramedullary canal of the bone using fluoroscopic guidance. The guide wire is then reamed to prepare the canal for the nail. The gamma nail is inserted over the guide wire and advanced into the canal until it reaches the fracture site. The proximal and distal locking screws are then inserted to secure the nail in place.
Complications
While the gamma nail is generally considered a safe and effective treatment option, it is not without its potential complications. Complications associated with the use of a gamma nail may include:
Malalignment or malrotation of the nail
Fracture of the nail or the bone
Nonunion or delayed union of the fracture
Infection
Hardware failure
Damage to surrounding structures, such as nerves or blood vessels
Outcomes
Numerous studies have evaluated the outcomes associated with the use of a gamma nail for the treatment of long bone fractures. Overall, the results have been positive, with high rates of fracture union, low rates of complications, and good functional outcomes reported. A meta-analysis of 22 studies found that the use of a gamma nail resulted in a 95% union rate and a 92% good or excellent functional outcome.
Conclusion
In conclusion, the gamma intramedullary nail is a popular and effective treatment option for long bone fractures. It offers numerous advantages over other fixation methods, including stable fixation, preservation of the biology of the fracture site, and early weight-bearing. While it is not without its potential complications, the overall outcomes associated with the use of a gamma nail are excellent.
Gamma Nail FAQs
1: How long does it take to recover after Gamma Nail surgery?
Recovery time after Gamma Intramedullary Nail surgery varies depending on the fracture type, fracture location (such as intertrochanteric or subtrochanteric fractures), patient age, bone quality, and overall health condition.
In general, most patients begin early mobilization shortly after surgery and can expect functional recovery and a return to daily activities within 3 to 6 months, provided there are no complications and rehabilitation protocols are followed.
2: Is a Gamma Nail suitable for all long bone fractures?
No. Although the Gamma Nail system is widely used for the fixation of proximal femoral and selected long bone fractures, it is not suitable for all fracture types.
The indication depends on factors such as fracture pattern, bone anatomy, patient condition, and surgeon assessment. Final implant selection should always be based on clinical evaluation and surgical judgment.
3: Is Gamma Nail insertion a painful procedure?
Gamma Nail insertion is performed under general or regional anesthesia, meaning patients do not experience pain during the surgical procedure itself.
Postoperative pain or discomfort may occur but is usually temporary and can be effectively managed with pain medication, rehabilitation, and proper postoperative care.
4: What are the possible complications of Gamma Nail surgery?
As with any orthopedic implant procedure, Gamma Nail surgery carries potential risks. Possible complications may include implant malalignment, delayed union or nonunion, infection, hardware failure, fracture around the implant, or injury to surrounding nerves or blood vessels.
Careful surgical technique, proper implant selection, and postoperative monitoring help minimize these risks.
5: Does a Gamma Intramedullary Nail need to be removed after fracture healing?
In most cases, a Gamma Intramedullary Nail does not require removal once the fracture has healed.
However, implant removal may be considered if the patient experiences persistent pain, implant-related irritation, or other complications. The decision should be made by the treating orthopedic surgeon based on clinical symptoms and imaging results.
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu