PFNA Intramedullary Nail: A Comprehensive Guide
In the field of orthopedics, the treatment of hip fractures remains a challenging task. While different surgical techniques have been developed over time, intramedullary nailing has emerged as a popular choice. The Proximal Femoral Nail Antirotation (PFNA) is a type of intramedullary nail that has gained significant attention due to its effectiveness in treating hip fractures. In this article, we will provide a comprehensive guide on PFNA intramedullary nail, covering everything from its design to the surgical procedure, post-operative care, and potential complications.
1. Introduction
Hip fractures are a common cause of morbidity and mortality among the elderly population. With the aging population, the number of hip fractures is expected to increase over time. The treatment of hip fractures is crucial, as it can significantly affect the quality of life of the patient. Intramedullary nailing has become a popular surgical technique for treating hip fractures due to its effectiveness and safety. Among the different types of intramedullary nails, PFNA intramedullary nail has gained significant attention.
2. What is PFNA Intramedullary Nail?
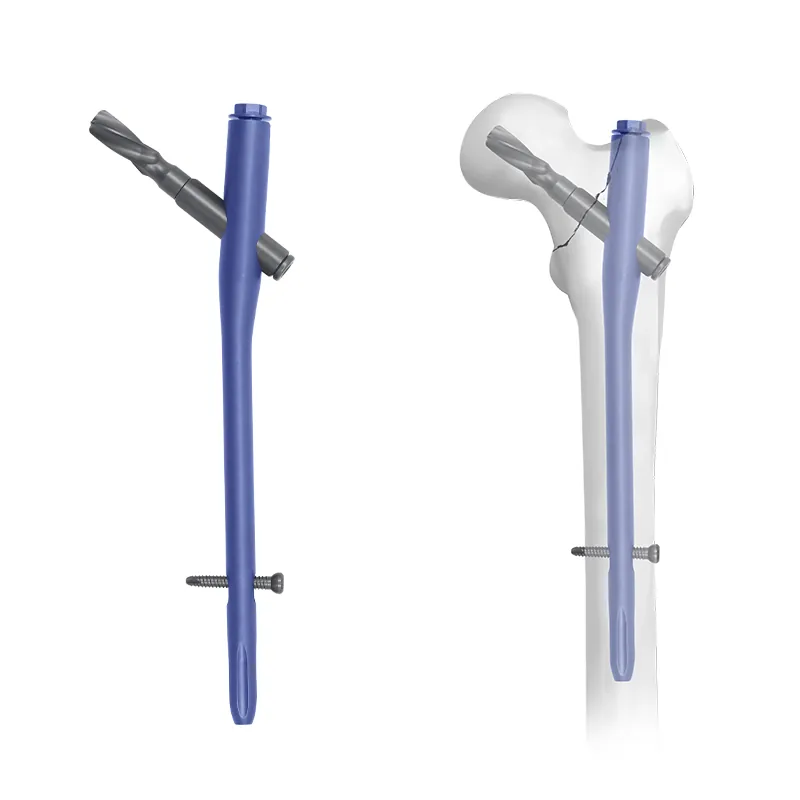
The PFNA intramedullary nail is a type of intramedullary nail that is designed to stabilize and fixate hip fractures. It is a proximally fixed, anterograde, and cephalomedullary device that is inserted through the proximal femoral canal. The PFNA nail provides stability to the fracture site by allowing load-sharing between the nail and the bone. It also reduces the risk of implant failure and loosening by providing anti-rotational stability.
3. PFNA Design and Features
The PFNA intramedullary nail is made up of titanium or titanium alloy, which is biocompatible and provides good strength and durability. The nail has a helical blade at the proximal end, which is designed to engage the femoral head and provide rotational stability. The blade also has an anti-rotation mechanism, which prevents rotation of the nail within the femur. The distal end of the nail has a locking mechanism, which allows for distal fixation and axial stability.
4. Indications for PFNA Surgery
The PFNA intramedullary nail is primarily used for the treatment of unstable intertrochanteric and subtrochanteric hip fractures. It is also used for the treatment of some femoral neck fractures. The decision to use PFNA intramedullary nail surgery depends on various factors such as patient age, bone quality, fracture type, and surgeon preference.
5. Surgical Technique of PFNA Intramedullary Nailing
The surgical technique of PFNA intramedullary nailing involves several steps. The procedure is typically performed under general or spinal anesthesia. The patient is positioned on a fracture table, and a fluoroscope is used to guide the nail insertion.The surgical technique of PFNA intramedullary nailing involves several steps. The procedure is typically performed under general or spinal anesthesia. The patient is positioned on a fracture table, and a fluoroscope is used to guide the nail insertion. The surgical approach involves a small incision made over the greater trochanter, which allows access to the proximal femur. The guide wire is then inserted through the incision and passed down the femoral canal under fluoroscopic guidance. The proximal femoral canal is then reamed to the appropriate size, and the PFNA intramedullary nail is inserted. The helical blade is inserted into the femoral head, and the locking mechanism is engaged in the distal femur to provide axial stability.
6. Post-operative Care after PFNA Surgery
After the PFNA intramedullary nail surgery, the patient is usually kept on bed rest for a few days. The affected limb is immobilized with a brace or cast for several weeks to promote healing. The patient is advised to avoid weight-bearing activities for a few months to allow for proper healing. Physical therapy is usually started early to improve joint range of motion and muscle strength.
7. PFNA Complications and Management
Like any surgical procedure, PFNA intramedullary nailing carries some risks and potential complications. These complications include infection, implant failure, nerve injury, blood vessel injury, and non-union. However, the overall complication rate of PFNA intramedullary nailing is relatively low. The management of these complications usually involves revision surgery or conservative treatment, depending on the severity of the complication.
8. PFNA vs. Other Intramedullary Nails
Compared to other types of intramedullary nails, the PFNA intramedullary nail has several advantages. One of the significant advantages of the PFNA nail is its anti-rotation mechanism, which provides rotational stability to the femoral head. It also allows for load-sharing between the nail and the bone, reducing the risk of implant failure. The PFNA nail is also relatively easy to insert and has a lower risk of complications.
9. Advantages of PFNA Intramedullary Nailing
PFNA intramedullary nailing has several advantages over other surgical techniques for treating hip fractures. One of the main advantages is its effectiveness in treating unstable intertrochanteric and subtrochanteric hip fractures. The PFNA nail also allows for early mobilization and shorter hospital stays compared to other surgical techniques. It also has a lower risk of implant failure and provides good functional outcomes.
10. Disadvantages of PFNA Intramedullary Nailing
While PFNA intramedullary nailing has several advantages, it also has some disadvantages. One of the main disadvantages is the potential risk of complications, such as implant failure, infection, and nerve injury. The PFNA nail is also relatively expensive compared to other surgical techniques.
11. PFNA Intramedullary Nail Outcomes and Success Rates
Studies have shown that PFNA intramedullary nailing has good outcomes and success rates in treating hip fractures. The success rates of PFNA nailing range from 70% to 90%, with good functional outcomes reported in most cases. The PFNA nail also has a low rate of revision surgery and implant failure.
12. PFNA in Geriatric Patients
Hip fractures are more common in the elderly population, and PFNA intramedullary nailing has emerged as a popular surgical technique for treating hip fractures in geriatric patients. The PFNA nail has been shown to have good outcomes in this population, with a low rate of complications and a shorter hospital stay.
13. Future Develop
The PFNA intramedullary nail has undergone several modifications since its introduction, with the aim of improving its effectiveness and reducing its risks. Some of the modifications include changes in the helical blade design, improvements in the locking mechanism, and modifications in the nail length and diameter. The development of new materials, such as titanium alloys and biodegradable materials, is also being explored to improve the performance of the PFNA nail.
14. Conclusion
In summary, the PFNA intramedullary nail is a popular surgical technique for treating hip fractures, especially in the elderly population. The PFNA nail provides good outcomes, with a low rate of complications and a shorter hospital stay. However, like any surgical procedure, it carries some risks and potential complications. The development of new materials and modifications in the nail design will likely improve the performance of the PFNA nail in the future.
FAQs
What is a PFNA intramedullary nail?
A PFNA intramedullary nail is a surgical implant used to treat hip fractures. It is inserted into the femoral canal and provides stability to the femoral head.
How is the PFNA intramedullary nail inserted?
The PFNA intramedullary nail is inserted through a small incision made over the greater trochanter. A guide wire is inserted into the femoral canal, and the canal is reamed to the appropriate size. The PFNA nail is then inserted, and the locking mechanism is engaged in the distal femur.
What are the advantages of the PFNA intramedullary nail?
The PFNA intramedullary nail has several advantages, including its anti-rotation mechanism, load-sharing properties, and ease of insertion. It is also effective in treating unstable hip fractures and allows for early mobilization.
What are the potential complications of PFNA intramedullary nailing?
The potential complications of PFNA intramedullary nailing include infection, implant failure, nerve injury, blood vessel injury, and non-union.
What is the success rate of PFNA intramedullary nailing?
The success rates of PFNA intramedullary nailing range from 70% to 90%, with good functional outcomes reported in most cases.
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu