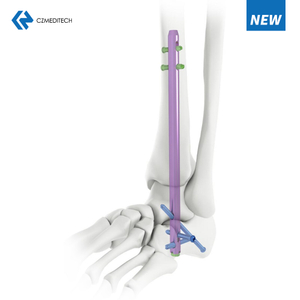
Intramedullary Tibial Nail System Overview for Tibial Shaft Fracture Fixation
The Tibial Intramedullary Nail System is designed for stable internal fixation of tibial shaft fractures using a minimally invasive intramedullary approach. By positioning the implant within the medullary canal, tibial nails provide axial and rotational stability while preserving surrounding soft tissues and periosteal blood supply.
This system is widely used in trauma surgery for both simple and complex tibial fractures, supporting early mobilization and predictable fracture healing outcomes.
Clinical Indications and Surgical Applications of Tibial Intramedullary Nails
Tibial intramedullary nails are indicated for a broad range of tibial fracture patterns, including:
Diaphyseal Tibial Shaft Fractures
Suitable for transverse, oblique, spiral, and comminuted fractures of the tibial shaft.
Proximal and Distal Tibial Fractures
Advanced locking configurations allow stable fixation near metaphyseal regions.
Open and Closed Tibial Fractures
Compatible with staged fixation strategies for both open and closed trauma cases.
Polytrauma and High-Energy Injuries
Provides strong biomechanical support in complex trauma scenarios requiring load-sharing fixation.
Design Features and Biomechanical Advantages of Tibial Nail Fixation Systems
The tibial nail system incorporates anatomical and biomechanical principles to enhance fixation stability and reduce complication risks.
Anatomically Contoured Nail Design
Optimized curvature ensures better canal fit and reduced stress concentration.
Load-Sharing Fixation Concept
Intramedullary positioning allows physiological load transfer, promoting callus formation.
Multi-Plane Locking Capability
Supports resistance to axial, torsional, and bending forces during rehabilitation.
Locking Options and Fixation Configurations for Proximal and Distal Tibia
Multiple locking options are available to accommodate varying fracture locations and bone quality.
Proximal Locking Options
Designed to control varus, valgus, and rotational forces in proximal tibial fractures.
Distal Locking Options
Ensures stable fixation in distal third tibial fractures, minimizing malalignment risks.
Static and Dynamic Locking Modes
Supports surgeon preference and fracture healing strategy.
Surgical Technique Compatibility and Instrumentation for Tibial Nailing Procedures
The tibial nail system is compatible with standardized intramedullary nailing techniques.
Guided Entry and Reaming System
Facilitates accurate nail insertion while reducing intraoperative variability.
Targeting Devices for Locking Screws
Improves locking accuracy and shortens surgical time.
Minimally Invasive Surgical Workflow
Supports reduced soft tissue trauma and faster postoperative recovery.
Materials, Surface Treatment, and Manufacturing Standards of Tibial Nails
Medical-Grade Titanium Alloy
Manufactured from high-strength titanium alloy to ensure biocompatibility and fatigue resistance.
Surface Finishing and Corrosion Resistance
Optimized surface treatment enhances wear resistance and long-term implant stability.
Precision Manufacturing
Produced under strict quality control to ensure dimensional accuracy and consistency.
Implant Sizes, Anatomical Matching, and Customization Options
The tibial nail system offers a comprehensive range of diameters and lengths to accommodate patient anatomy.
Multiple Length and Diameter Options
Ensures optimal canal filling and fixation strength.
Left and Right Anatomical Compatibility
Designed for universal application without side limitation.
OEM and Custom Configuration Support
Customization available based on regional or clinical requirements.
Quality Assurance, Certifications, and Regulatory Compliance
All tibial intramedullary nail systems are manufactured under certified quality management systems.
This ensures product safety, reliability, and regulatory acceptance in global markets.
OEM / ODM Supply Capability and Global Distribution Support
The tibial nail system is available for OEM and ODM cooperation.
Private labeling and branding support
Customized packaging and instrument sets
Stable production capacity and global logistics coordination
This enables distributors and partners to expand orthopedic trauma portfolios efficiently.
FAQs About Tibial Intramedullary Nail Systems
Q: What fractures are tibial intramedullary nails used for?
Tibial nails are primarily used for tibial shaft fractures, including simple, comminuted, proximal, and distal fractures.
Q: Are tibial nails suitable for minimally invasive surgery?
Yes. Tibial intramedullary nailing is a minimally invasive technique that preserves soft tissue and blood supply.
Q: What materials are used in tibial nail systems?
Most tibial nails are manufactured from medical-grade titanium alloy for strength and biocompatibility.
Q: Can this tibial nail system support early weight bearing?
With appropriate fracture stability and surgical technique, early mobilization is often achievable.
Q: Is OEM customization available for tibial nail systems?
Yes. OEM and ODM services are available for implant dimensions, packaging, and branding.
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu