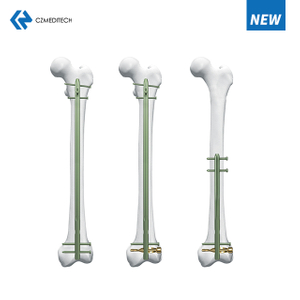
Femur (Femoral) Nail Uses
Comminuted fractures
Segmental fractures
Fractures with bone loss
Proximal and distal fractures
Nonunions
Subtrochanteric fractures
Intertrochanteric fractures
How to use Femoral Intramedullary Nail?
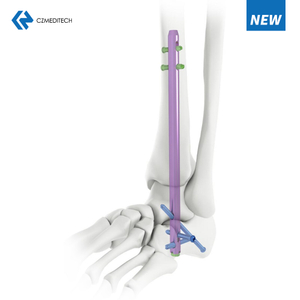
Femoral intramedullary nails (IM nails) are surgical implants that are used to treat fractures of the femur, the long bone in the thigh. The procedure involves inserting the nail into the intramedullary canal (the hollow center) of the femur, and securing it in place with screws or locking mechanisms at the ends of the nail.
Here are the general steps for using a femoral intramedullary nail:
Anesthesia: The patient is given anesthesia, either general or regional (spinal or epidural).
Incision: The surgeon makes an incision near the hip or knee joint, depending on the location of the fracture.
Preparation of the femur: The surgeon creates an opening in the bone to access the intramedullary canal.
Insertion of the nail: The nail is inserted into the canal and advanced to the fracture site.
Alignment: The surgeon uses fluoroscopy (real-time X-ray) to confirm that the nail is properly aligned with the bone and the fracture has been reduced.
Fixation: Screws or locking mechanisms are used to secure the nail in place at the ends of the bone.
Closure: The incision is closed with sutures or staples, and a sterile dressing is applied.
Postoperative care includes pain management, physical therapy, and follow-up X-rays to monitor the healing process.
It's important to note that the specific steps and techniques may vary depending on the type of femoral intramedullary nail being used, as well as the surgeon's preferences and experience. The procedure should only be performed by a trained and experienced orthopedic surgeon.
Femur (Femoral) Nail Contraindications
The physician’s education, training and professional judgement must be relied upon to choose the most appropriate device and treatment.
Conditions presenting an increased risk of failure include:
Any active or suspected latent infection or marked local inflammation in or about the affected area.
Compromised vascularity that would inhibit adequate blood supply to the fracture or the operative site.
Bone stock compromised by disease, infection or prior implantation that can not provide adequate support and/or fixation of the devices.
Material sensitivity, documented or suspected.
Obesity. An overweight or obese patient can produce loads on the implant that can lead to failure of the fixation of the device or to failure of the device itself.
Patients having inadequate tissue coverage over the operative site.
Implant utilization that would interfere with anatomical structures or physiological performance.
Any mental or neuromuscular disorder which would create an unacceptable risk of fixation failure or complications in postoperative care.
Other medical or surgical conditions which would preclude the potential benefit of surgery.
How to Buy High Quality Femoral Intramedullary Nail?
If you are looking to buy a high-quality femoral intramedullary nail, here are some general guidelines:
Research and select a reputable manufacturer or supplier of medical devices. Look for companies that have a proven track record of producing high-quality implants and instruments.
Check for certification and regulatory compliance. Make sure that the manufacturer or supplier has obtained the necessary certifications and approvals from regulatory bodies such as the FDA (in the US), CE (in the EU), or ISO (International Organization for Standardization).
Verify the product specifications. Check that the femoral intramedullary nail meets the required specifications in terms of size, shape, and material.
Consider the features of the implant. Look for features such as locking mechanisms, adjustable angles, and anti-rotation capabilities, which may provide additional benefits for the patient and surgeon.
Consult with a medical professional. Before making a purchase, it is important to consult with a qualified orthopedic surgeon or other medical professional to ensure that the femoral intramedullary nail is suitable for your specific needs.
Ensure proper storage and handling. After purchasing the implant, make sure to store and handle it according to the manufacturer's instructions to ensure that it remains in optimal condition for use in surgery.
About CZMEDITECH
CZMEDITECH is a medical device company that specializes in the production and sale of high-quality orthopedic implants and instruments, including intramedullary nails. The company has over 14 years of experience in the industry and is known for its commitment to innovation, quality, and customer service.
When purchasing intramedullary nails from CZMEDITECH, customers can expect products that meet international standards for quality and safety, such as ISO 13485 and CE certification. The company uses advanced manufacturing technologies and strict quality control processes to ensure that all products are of the highest quality and meet the needs of surgeons and patients.
In addition to its high-quality products, CZMEDITECH is also known for its excellent customer service. The company has a team of experienced sales representatives who can provide guidance and support to customers throughout the purchasing process. CZMEDITECH also offers comprehensive after-sales service, including technical support and product training.
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu