How to Choose the Right Orthopedic Supplier — Indonesia Hospital Expo Insights
Event: Indonesia Int'l Hospital Expo 2025 | Date: September 25–28, 2025 | Booth: Hall 2-428 | Location: Indonesia Convention Exhibition (ICE), Tangerang, Banten
1. Why We Chose to Attend Indonesia Hospital Expo 2025
In the medical device industry, building genuine trust always starts with personal connection. That belief inspired CZMEDITECH's participation in the Indonesia International Hospital Expo 2025. As one of the fastest-growing healthcare markets in Southeast Asia, Indonesia represents a vital link between innovation and accessibility in orthopedics.
Our goal was to present high-quality orthopedic implants and surgical solutions that meet global CE and ISO13485 standards — and to demonstrate how precision engineering from China can empower hospitals, distributors, and surgeons across the region.
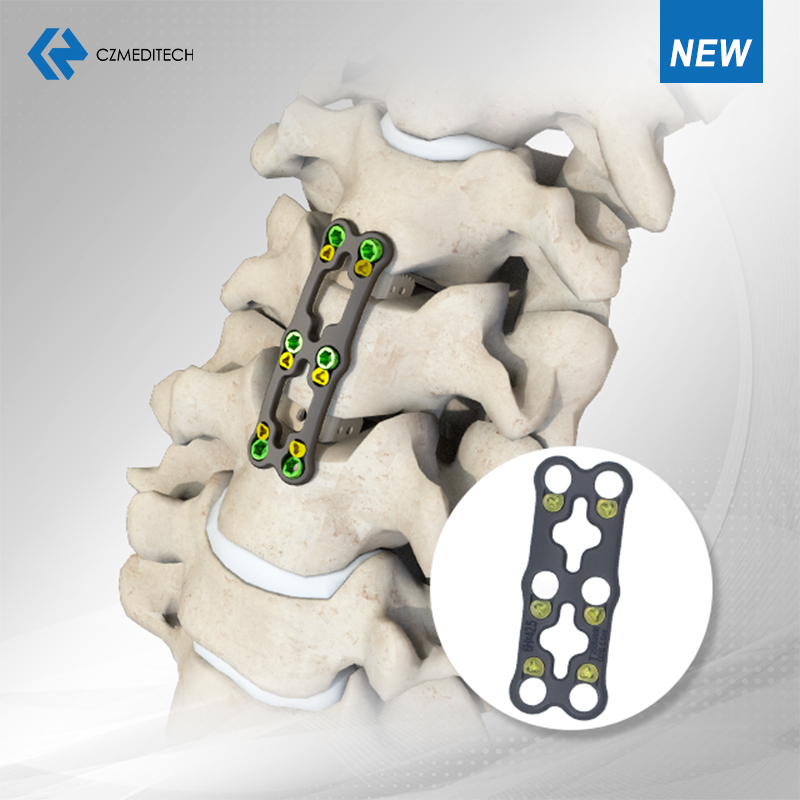
2. Highlights from Our Booth at Hall 2-428
At Booth Hall 2-428, we unveiled a range of CZMEDITECH's core systems — from spinal fixation and anterior cervical plates to expandable titanium cages and fibular intramedullary nails. Each product embodies our focus on safety, stability, and surgical efficiency.
Many surgeons and buyers spent time examining our devices in person, discussing thread geometry, surface finishing, and material durability. Their feedback — often a simple, genuine nod of approval — reminded us that real craftsmanship still speaks louder than slogans.
3. Key Outcomes and Market Insights
The exhibition opened the door to meaningful conversations with healthcare professionals from Indonesia, Thailand, Malaysia, and the Middle East. Visitors were particularly interested in our integrated manufacturing capabilities — from R&D and CNC machining to anodizing and surface treatment.
We learned that today's buyers value not only pricing but also delivery reliability, certification transparency, and after-sales responsiveness. These insights will guide our continued partnerships in Southeast Asia, where local collaboration and technical reliability truly matter.
4. The Ultimate Guide: How to Choose the Right Orthopedic Supplier
Based on years of industry experience and insights from this year's exhibition, we've summarized three essential principles for identifying a trustworthy orthopedic supplier:
Certification First: Always verify CE, ISO13485, or FDA compliance — they are the foundation of product quality and patient safety.
Ask Beyond the Brochure: Inquire about production processes, material sourcing, and post-sale service. Reliable suppliers are open about their operations.
Evaluate Professionalism: True experts go beyond presentation — they provide technical suggestions tailored to your market's needs.
Pro Tip: Look for suppliers who continue communication long after the exhibition ends — consistency signals commitment.
5. Featured Products
Spinal Fixation System — Innovative stabilization with precision alignment.
Anterior Cervical Plate System — Engineered for optimal fusion outcomes.
PEEK Cage — Promotes bone growth and long-term biocompatibility.
Fibular Intramedullary Nail — Designed for trauma and reconstructive applications.
![1 1]()
Cervical Extendable Titanium Mesh Cage
Implant Material: Titanium Alloy Potential Applications:Cervical corpectomy defects reconstruction Reconstruction after vertebral body tumor resection Severe vertebral body fractures requiring corporectomy
![2 2]()
Titaium Mesh
Implant Material: Commercially Pure Titanium or Titanium Alloy Potential Applications:Cranioplasty for skull defects Reconstruction of orbital wall fractures Mandibular reconstruction Reconstruction of maxillofacial bone defects
![3 3]()
Cranial Clamps Skull Lock
Implant Material: Titanium Alloy Potential Applications:Fixation of bone flaps in neurosurgical craniotomies Pediatric craniofacial surgery
![4 4]()
Distal Tibial Intramedullary Nail
Our newly developed Distal Femoral Nail (DFN) and Fibular Intramedullary Nail deliver advanced solutions for lower limb fracture treatment, emphasizing reduced soft tissue injury and accelerated recovery.
6. Frequently Asked Questions (FAQ)
Q1: Are your products internationally certified?
A1: Yes, all CZMEDITECH orthopedic implants are CE and ISO13485 certified.
Q2: Do you offer customized orthopedic solutions?
A2: Absolutely. We provide customization in dimensions, angles, and surface treatment to match clinical needs.
Q3: How can I become a CZMEDITECH distributor?
A3: Simply visit czmeditech.com/contact-us and submit your details. Our regional manager will reach out within 24 hours.
7. Final Thoughts
Exhibitions are more than product showcases — they are moments of connection. At Indonesia Hospital Expo 2025, we listened to surgeons' insights, shook hands with future partners, and reaffirmed our mission: bringing reliable orthopedic innovations from China to the world.
With each event, CZMEDITECH continues to expand globally, committed to advancing surgical outcomes and empowering medical professionals with precision-engineered implants.
![1080-2 1080-2]()
Conclusion and Future Outlook
Our successful participation in Indonesia Int’l Hospital Expo 2025 marks a significant step forward in CZMEDITECH’s global growth and Southeast Asia market strategy.
We will continue to innovate and develop orthopedic implant solutions that meet evolving clinical needs, applying the valuable feedback and market insights gained from the exhibition to enhance our R&D, product design, and regional marketing approaches.
As we strengthen collaborations with hospitals, distributors, and medical professionals across Indonesia and neighboring ASEAN countries, our mission remains clear — to deliver safer, smarter, and more effective orthopedic solutions that improve surgical outcomes and enhance patients’ quality of life worldwide.
Uni-C Standalone Cervical Cage
Posterior Cervical Screw System (New)
Anterior Cervical Spine Fixation System
Cranial clamps skull Lock
CERVICAL EXTENDABLE TITANIUM MESH CAGE
Distal Tibial Intramedullary Nail
Expert Fibular Intramedullary Nail
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu