MEDICAL FAIR THAILAND
MEDICAL FAIR THAILAND is one of Southeast Asia's most influential medical exhibitions, attracting thousands of professionals and exhibitors from over 40 countries worldwide. The 2025 edition, held from September 10 to 12 in Bangkok, focused on digital health innovations—including AI-assisted diagnostics and wearable devices—as well as traditional medical equipment.
For CZMEDTITECH, participating in this prestigious event provided a valuable platform to demonstrate comprehensive orthopedic solutions and advance global healthcare progress.
CZMEDTITECH at the Exhibition
As a leading manufacturer of orthopedic implants, CZMEDTITECH’s presence at MEDICAL FAIR THAILAND 2025 was a strategic move to strengthen its footprint in the ASEAN market, which boasts over 400 million people and a rapidly growing medical tourism sector.
Our participation enabled us to connect with distributors, surgeons, and hospital procurement teams from Thailand, Vietnam, Singapore, Indonesia, and beyond, fostering relationships for future collaboration.
This aligns with Thailand’s goal to become a global healthcare and wellness hub under its "National Medical Hub Strategy (2025–2034)."
Key Product Lines and Innovations
At our booth, we displayed a comprehensive product portfolio, with special emphasis on latest R&D achievements designed to meet the evolving needs of surgeons and patients:
Locking Plate Series: Our new Femoral Neck System (FNS) offers a novel solution for fracture stabilization, featuring enhanced biomechanical stability and minimally invasive application.
Implant Material:Titanium Alloy
Plate Design: Low-profile, pre-contoured to fit the lateral femoral cortex.
Potential Applications:
Femoral neck fractures (Pauwels classification types II and III)
Basicervical femoral neck fractures
Selected petrochanteric fractures
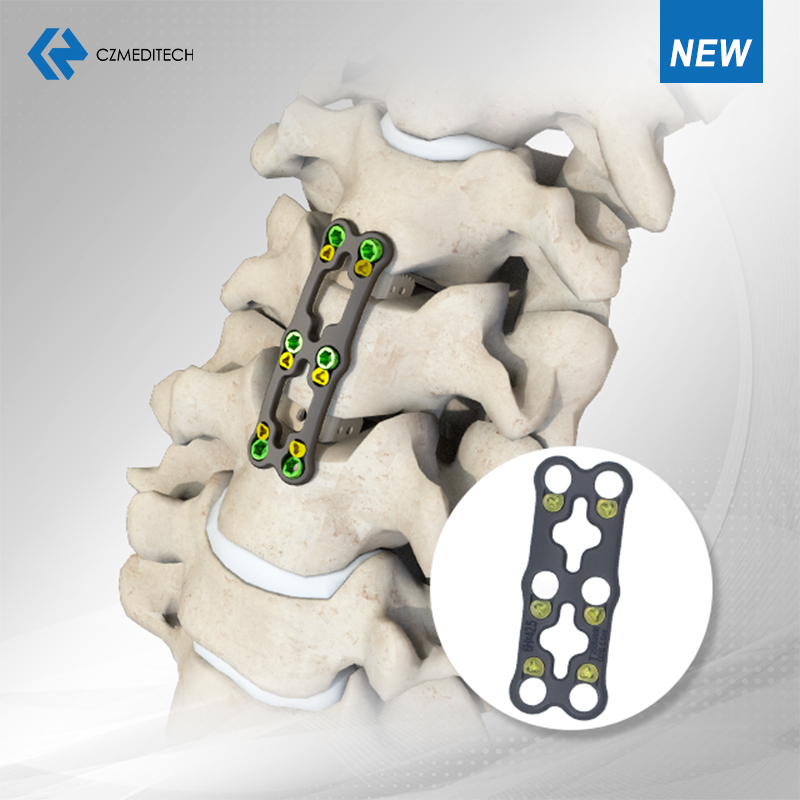
Spine Solutions: Our expanded spinal portfolio includes Minimally Invasive Systems (MIS), Interbody Fusion Cages, Posterior Cervical Screw-Rod Systems, Anterior Cervical Plates, and 2-Screw/4-Screw Fusion Devices—all engineered to improve surgical precision and patient outcomes.
Implant Material: Titanium Alloy (Ti-6Al-4V)
Potential Applications:
Anterior Cervical Discectomy and Fusion (ACDF)
Cervical corpectomy reconstruction
Treatment of cervical degenerative disc disease, trauma, tumors, or deformities
Implant Material: Titanium Alloy
Potential Applications:
Posterior cervical fusion (C1-C2 and subaxial cervical spine)
Stabilization of cervical fractures and dislocations
Treatment of cervical instability due to degeneration, trauma, or deformity
Occipitocervical fusion
Implant Material: PEEK or Titanium Alloy.
Potential Applications:
Anterior Cervical Discectomy and Fusion (ACDF)
Treatment of single or multi-level cervical DDD
Cervical Extendable Titanium Mesh Cage
Implant Material: Titanium Alloy
Potential Applications:
Cervical corpectomy defects reconstruction
Reconstruction after vertebral body tumor resection
Severe vertebral body fractures requiring corporectomy
Titaium Mesh
Implant Material: Commercially Pure Titanium or Titanium Alloy
Potential Applications:
Cranioplasty for skull defects
Reconstruction of orbital wall fractures
Mandibular reconstruction
Reconstruction of maxillofacial bone defects
Nail Diameter & Length:
Diameter: 3.0 mm, 4.0 mm
Length: 130 mm - 230 mm
Anatomy:
Tibia
These products are manufactured using high-quality materials such as titanium and cobalt-chromium alloys, ensuring durability and biocompatibility.
Customer Engagement and Collaborative Moments
The exhibition served as a dynamic platform for in-depth exchanges with both new and existing clients. Live demonstrations of product applications and benefits—especially newly launched items—drew significant attention and positive feedback.
The enthusiastic response from global medical professionals reinforced our confidence in the market potential of these innovative solutions.
Photographs taken during the event, including interactions with surgeons and distributors, reflected a spirit of partnership and a shared commitment to improving patient care.
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Client Engagement
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Client Engagement
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Client Engagement
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Client Engagement
Conclusion and Future Outlook
Our successful participation in MEDICAL FAIR THAILAND 2025 marks an important milestone in CZMEDTITECH’s global expansion strategy.
We will continue to innovate and develop products that address unmet clinical needs, leveraging insights gained from the exhibition to refine our R&D and marketing strategies.
By strengthening partnerships and exploring new opportunities across Southeast Asia and beyond, we aim to deliver better outcomes for patients worldwide.
Uni-C Standalone Cervical Cage
Posterior Cervical Screw System (New)
Anterior Cervical Spine Fixation System
Cranial clamps skull Lock
CERVICAL EXTENDABLE TITANIUM MESH CAGE
Distal Tibial Intramedullary Nail
Expert Fibular Intramedullary Nail
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu