FNS (Femoral Neck System)
Product Overview of FNS Femoral Neck System
What Is the FNS Femoral Neck System for Femoral Neck Fractures?
The FNS system is a medical device intended to temporarily fix, correct or stabilize fractures in the femoral neck region. It consists of an anatomically contoured locking plate, a central fixation bolt, anti-rotation screws, and dedicated instrumentation to support precise placement with minimal soft tissue disruption. CZMeditech The minimally invasive design allows smaller incisions, reduced trauma to surrounding tissues, and a shorter perioperative recovery period compared to traditional open procedures.
Intended Use and Clinical Application of FNS Hip Fixation System
Indications for Femoral Neck Fracture Fixation
Femoral neck fractures requiring stable internal fixation
Fracture patterns where preservation of the femoral head is desired
Adult patients where minimal invasive internal fixation is indicated
These indications are consistent with the intended use defined in the product description.
Contraindications of FNS Orthopedic Implant
The FNS system should not be used where internal fixation cannot provide adequate stabilization due to severely compromised bone quality or in active infection at the surgical site. Specific contraindications may include:
Proper patient assessment and surgical judgment should guide device usage.
Warnings and Precautions for Hip Fracture Internal Fixation
Clinical decisions must consider patient-specific factors including bone quality, comorbidities, and overall fracture complexity. Surgeons must optimize implant selection and placement to minimize the risks associated with mechanical failure or postoperative complications. Surgical imaging and intraoperative monitoring are recommended to ensure correct positioning and alignment.
Additionally, this system should not be used for cases where there is a high incidence of:
Possible Adverse Events of Femoral Neck Internal Fixation System
As with any internal fixation device and surgical procedure, risks may occur. Common potential adverse events include, but are not limited to:
Incision site infection
Implant loosening or breakage
Delayed union or nonunion
Soft tissue irritation
Temporary or permanent nerve or vessel injury
Allergic reactions to implant material
Your orthopedic surgeon will discuss potential risks and measures to minimize complications before surgery.
![DFN Distal Femur Intramedullary Nail (Spiral Blade Screw Type)]()
Key Features and Benefits of FNS Femoral Neck Fixation System
Technical Specifications of FNS Femoral Neck Fixation System
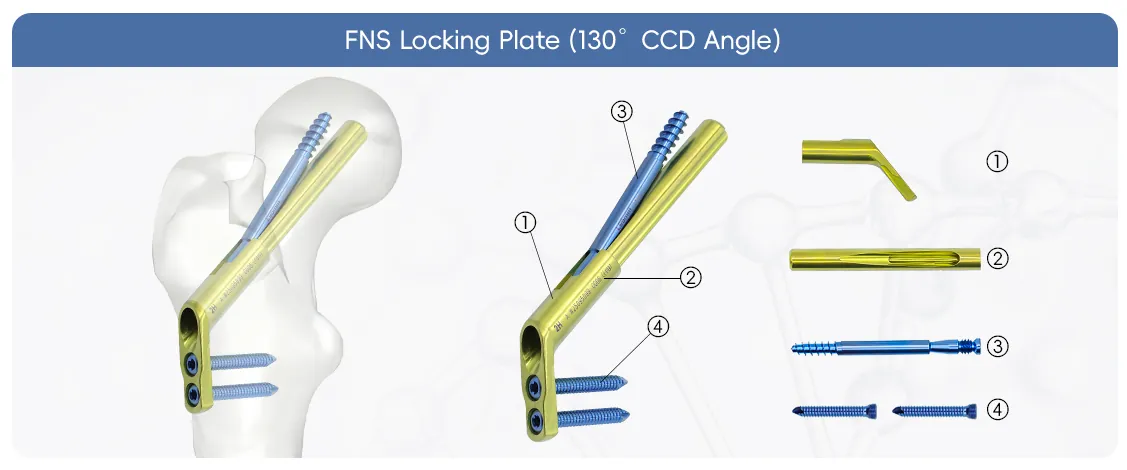
The FNS (Femoral Neck System) Locking Plate is an innovative implant designed for the fixation of femoral neck fractures. It represents a paradigm shift from traditional multiple screw constructs by offering a standardized, simplified implant with angular stability.
The 5.0mm Locking Screw is specifically designed to secure the FNS Locking Plate to the femoral shaft.
This is the core implant combination that provides the unique dynamic compression and anti-rotation function of the FNS system.
The Bolt: This is the main implant that is inserted through the plate and into the femoral head.
The Antirotation Screw: Placed parallel to the main bolt, this screw primarily functions to prevent rotation of the femoral head fragment.
The FNS Instrument Set (3300-04) is a comprehensive and meticulously designed surgical toolkit that ensures precise, efficient, and reproducible implantation of the FNS system.
| name |
specifications |
REF(titanium alloy) |
FNS Locking Plate (130° CCD angle) (Use 5.0 Locking Screw) |
1 hole |
3300-0101 |
| 2 holes |
3300-0102 |
| FNS Bolts+Antirotation Screws |
75mm |
3300-0201 |
| 80mm |
3300-0202 |
| 85mm |
3300-0203 |
| 90mm |
3300-0204 |
| 95mm |
3300-0205 |
| 100mm |
3300-0206 |
| 105mm |
3300-0207 |
| 110mm |
3300-0208 |
| 115mm |
3300-0209 |
![DFN Distal Femur Intramedullary Nail (Spiral Blade Screw Type)]()
Product Images and Actual Photos of FNS Orthopedic System
![DFN Distal Femur Intramedullary Nail (Spiral Blade Screw Type)]()
Clinical Knowledge and Educational Resources on FNS System
FNS (Femoral Neck System): An Overview
The femoral neck is the part of the thigh bone that connects to the hip joint. Injuries to this area can be severe and may require surgery. One surgical option is the Femoral Neck System (FNS), a medical device used to treat fractures of the femoral neck. In this article, we will provide an overview of FNS, including its benefits, risks, and recovery process.
What is FNS?
FNS is a medical device designed to provide fixation and stabilization of femoral neck fractures. The device consists of a plate and screws, which are used to stabilize the broken bone. The system is designed to be minimally invasive, which means that it requires smaller incisions and causes less trauma to the surrounding tissue compared to traditional open surgery.
Benefits of FNS
The use of FNS provides several benefits, including:
Faster recovery: The minimally invasive nature of the procedure means that patients can recover more quickly than with traditional open surgery.
Reduced pain: The smaller incisions and less traumatic procedure can result in less post-operative pain.
Lower risk of complications: FNS has a lower risk of complications such as infection, nerve damage, and blood loss.
Improved mobility: FNS can help restore mobility and function to the affected area more quickly than traditional open surgery.
Risks and complications
As with any surgical procedure, there are potential risks and complications associated with the use of FNS. These include:
Infection: There is a risk of infection at the site of the incision or around the screws used to attach the plate.
Implant failure: The plate may loosen or break over time, requiring additional surgery.
Nerve or blood vessel damage: The surgical procedure can damage nerves or blood vessels in the surrounding area, leading to numbness or tingling in the leg or foot.
Allergic reaction: Some patients may have an allergic reaction to the metal used in the plate.
Your orthopedic surgeon will discuss these risks and complications with you prior to the procedure and will take steps to minimize the risk of complications.
Recovery process
After the procedure, you will be instructed to keep weight off the affected leg for a period of time. You may be given crutches or a walker to assist with mobility. Physical therapy may also be prescribed to help restore strength and function to the affected leg. Recovery time can vary depending on the extent of the injury and the individual patient, but in general, it takes several weeks to a few months to fully recover.
Frequently Asked Questions About FNS Femoral Neck System
What is the FNS Femoral Neck System used for?
The FNS (Femoral Neck System) is used for internal fixation of femoral neck fractures. It is designed to stabilize the fracture, provide angular stability, and support bone healing while preserving the femoral head.
What types of femoral neck fractures are indicated for the FNS system?
The FNS system is indicated for femoral neck fractures that require stable internal fixation, particularly undisplaced or minimally displaced fractures, and cases where femoral head preservation is clinically desired.
How is the FNS system different from traditional cannulated screw fixation?
Compared with traditional cannulated screws, the FNS system provides improved angular and rotational stability through a combination of a fixation bolt, anti-rotation screw, and locking plate, helping to reduce the risk of fixation failure.
Is the FNS system suitable for minimally invasive surgery?
Yes. The FNS Femoral Neck System is designed to support minimally invasive surgical techniques, allowing smaller incisions, reduced soft tissue disruption, and potentially faster postoperative recovery.
What materials are used in the FNS Femoral Neck System?
The FNS system is manufactured from medical-grade titanium alloy, which offers high strength, corrosion resistance, and biocompatibility for orthopedic implantation.
Can the FNS system provide controlled compression at the fracture site?
Yes. The design of the FNS system allows controlled dynamic compression at the fracture site, which may help promote fracture healing while minimizing excessive femoral neck shortening.
What are the main components of the FNS Femoral Neck System?
The system consists of an anatomically contoured locking plate, a central fixation bolt, anti-rotation screws, 5.0 mm locking screws, and a dedicated instrument set for accurate implantation.
What are the possible risks or complications associated with the FNS system?
Potential risks include infection, delayed union or nonunion, implant loosening or breakage, femoral head necrosis, and the need for revision surgery. Proper patient selection and surgical technique are essential to reduce these risks.
Is the FNS system suitable for patients with osteoporosis?
In patients with severe osteoporosis, fixation stability may be compromised. The suitability of the FNS system should be carefully evaluated by the surgeon based on bone quality and fracture characteristics.
Does the FNS system require special surgical instruments?
Yes. The FNS system uses a dedicated instrument set (Model 3300-04) designed to support accurate guide wire placement, reaming, and controlled implant insertion.
Is the FNS Femoral Neck System supplied sterile?
The FNS system is supplied non-sterile and must be cleaned and sterilized according to standard hospital protocols before clinical use.
Can CZMEDITECH provide OEM or distributor support for the FNS system?
Yes. CZMEDITECH offers OEM and ODM services, technical support, and distributor cooperation for the FNS Femoral Neck System. For detailed information, consultation with CZMEDITECH orthopedic experts is recommended.
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu