RECTUS MEDICA THAILAND
MEDICA FAIR THAILAND una est ex exhibitionibus medicinae Southeast Asiae gravissimarum, mille doctorum et exhibitorum e super 40 terrarum terrarum orbe trahentium. Editio 2025, a die X Septembris ad XII in Bangkok habita, in innovationibus digitali sanitatis intentus est, cum AI diagnostica et machinas inexplicabiles adiuvavit, necnon apparatum medicorum traditum.
Pro CZMEDTITECH, huius prestigiosi eventus participatione praebuit suggestum perutile ad solutiones comprehensivas orthopaedicas demonstrandas et ad global curis progressum promovendum.
CZMEDTITECH ad Exhibition
Ut primarius opifex orthopaedicae implantationis, CZMEDTITECH praesentiam apud MEDICA FAIR THAILAND 2025 opportuna fuit motus ad confirmandum suum vestigium in foro ASEAN, quod super 400 miliones hominum gloriatur et in regione voluptuaria medicinae celeriter crescens.
Nostra participatio nos permisit cum distributoribus, chirurgis, chirurgis, et hospitalis procurationis iunctionibus ex Thailandia, Vietnam, Singapore, Indonesia coniungere, et ultra fovere relationes ad collaborationem futuram.
Hoc adsimilat cum proposito Thailandiae ut fiat centrum valetudinis globalis et commoditatis sub eius centrum 'Medicinae Hub Strategy Nationale (2025-2034).
Key Product Lines and Innovations
In nostra umbraculo portfolio producto comprehensivo demonstravimus, cum praesertim res gestas R&D recentissimas quae ad necessitates chirurgorum et aegrotorum occurrendas destinati sunt;
Obstructio Plate Series: Nostra nova Systema Femoralis Colli (FNS) novam solutionem fracturae stabilizationis praebet, featuratio stabilitatis biomechanicae aucta et applicationis minime incursivae.
Implant Material: Titanium Alloy
Plate Design: Low-profile, prae-contoured aptare cortex femoris lateralis.
Applications potentia:
Fracturae colli femoris (Pauwels genera II et III)
Fracturae collum femoris fundamentalis
Electus fracturae petrochantericae
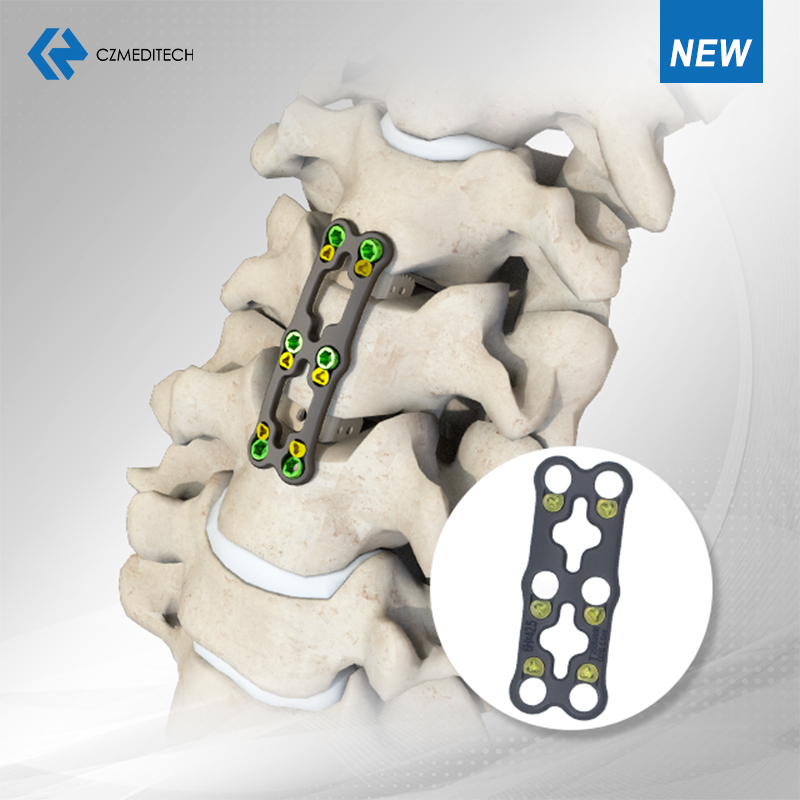
Solutio spinae: Nostrae portfolio dilatato spinae minimaliter Invasivae Systems (MIS), Fusion Cavea Intercorpus, Systema Cervicale Posterioris Cochleae, Plates Cervicalis Anterius, et 2-Screw/4-Screw Fusion machinae — omnes machinati sunt ad meliorem chirurgicam praecisionem et patientes exitus.
Materiam implantare: Titanium Alloy (Ti-6Al-4V)
Applications potentia:
Discectomy Cervical Anterius et Fusion (ACDF)
Reconstructione cervicali corpectomy
Curatio discus verticalis degenerativi morbus, trauma, tumores, vel deformitates
Implant Material: Titanium Alloy
Applications potentia:
Fusio cervicalis posterior (C1-C2 et spina cervicali subaxialis)
Stabilitas fracturae cervicales et luxationes
Curatio instabilitatis cervicis ob degenerationem, trauma, vel deformitatem
Fusio occipitocervicalis
Materiam implantare: PEEK vel Titanium Alloy.
Applications potentia:
Discectomy Cervical Anterius et Fusion (ACDF)
Curatio unius vel multi gradus ceruicis DDD
Titanium Mesh Cage
Implant Material: Titanium Alloy
Applications potentia:
Corpectomiae cervicales defectus refectionis
Reconstruction post tumorem corporis vertebrarum resection
Fracturae corporis vertebrales graves requirunt corporectomy
Titaium Mesh
Implant Material: Commercially Pure Titanium seu Titanium Alloy
Applications potentia:
Cranioplasty ad cranii vitia
Reconstructio fracturae muri orbitalis
Mandibulae refectionem
Reconstructio vitiorum ossis maxillofacialis
Clavus Diameter & Longitudo:
Diameter: 3.0 mm, 4.0 mm
Longitudo: 130 mm - 230 mm
Anatomia:
Tibia
Haec producta fabricantur utentes materiae qualitatem, ut titanium et chromium admixtum, ad vetustatem et biocompatibilitatem praestandam.
Customer cura et Collaborative Momenta
Exhibitio suggestum dynamicum pro altissimis commutationibus cum clientibus novis et existentibus servivit. Vive demonstrationes applicationum productorum et emolumenti - praesertim recentium rerum emissarum - notabilem attentionem hauserunt et opiniones positivas.
Alacer responsio e medicis professionalibus globalis fiduciam nostram in foro potentiae harum solutionum porttitorrum roboravit.
Imagines in eventu captae, inter interactiones cum chirurgis et distributoribus, spiritum societatis et munus communicatum ad patientiam colendam curaverunt.
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Clientem Engagement
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Clientem Engagement
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Clientem Engagement
![lQDPJwjlORYoo23NCxPNDYewCm4Zh7LuXHUIYFin61U-AA_3463_2835]()
Clientem Engagement
Conclusio et Future Outlook
Nostra felix participatio in MEDICA Fair THAILAND MMXXV notat magnum lapidem in CZMEDTITECH dilatatio globalis consilii.
Perge ad innovare et evolvere res quae necessarias clinicas inscriptioni incomitatas habent, pervestigationes leves ab exhibitione factas ad nostra R&D excolendas et negotia mercatoria consilia.
Societates confirmando et occasiones novas per Asiam orientalem et ultra explorando, meliores eventus pro aegris terrarum spatio liberare contendimus.
Uni-C Standalone Cervical Cage
Posterior Cochlea Systema Cervical (New)
Spina Cervicalis anterius systematis fixationis
Distal Tibial Intransmedullary Nail
Peritus Fibularis intramedullaris Nail
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu