Successful Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System
Case Overview
At Tlalixtac de Cabrera State Hospital, Mexico, a distal radius fracture surgery was successfully performed using a VA Distal Radius Locking Plate provided by CZMEDITECH, a leading orthopedic implant manufacturer from China.
The operation, led by Dr. José Alfredo Rios Melchor, achieved precise anatomical alignment and rapid patient recovery — highlighting how modern trauma fixation systems are improving outcomes across Latin America.
Case Description
A 53-year-old patient sustained a radius fracture near the wrist after a fall. The medical team opted for internal fixation using a variable angle distal radius locking plate, ensuring anatomical reduction and early mobilization.
Preoperative Image
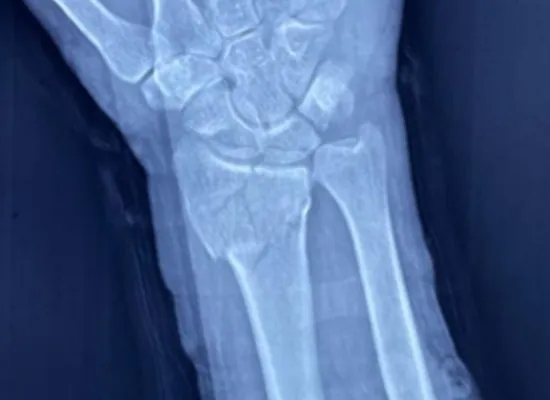
This case demonstrates how locking plate fixation can restore joint stability and function, especially in complex intra-articular fractures of the distal radius.
Postoperative Evaluation and Case Highlights
The surgery was efficient and completed with professional precision using CZMEDITECH orthopedic instruments.
Postoperative evaluation revealed excellent alignment, stable fixation, and early joint motion.
![Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System2 Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System2]()
![Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System3 Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System3]()
Implant Used:
VA Distal Radius Locking Plate (25.5 mm, 2-hole)
Postoperative Image
![Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System4 Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System4]()
This distal radius plate surgery shows how biocompatible titanium alloy implants contribute to bone healing and minimize irritation to surrounding soft tissues.
Instruments Used
![Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System5 Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System5]()
![Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System6 Distal Radius Fracture Surgery in Mexico — Using CZMEDITECH VA Locking Plate System6]()
The instruments included bone reduction forceps, drill guides, and screwdrivers specially designed for radius fracture fixation — ensuring high precision during the surgical process.
Note: The hospitals and doctors mentioned are pseudonyms.
Clinical Insight: The Importance of Distal Radius Locking Plate Fixation
Distal radius fractures are among the most common upper-limb injuries worldwide. Stable fixation with a variable angle distal radius plate is critical to maintain wrist motion and prevent postoperative complications.
The CZMEDITECH VA Locking Plate System offers optimized screw trajectory, high pull-out strength, and compatibility with minimally invasive surgical techniques. It's widely used for osteoporotic bone, comminuted fractures, and post-traumatic deformity correction.
By integrating precision engineering and biocompatible titanium, CZMEDITECH provides reliable solutions for orthopedic surgeons globally, bridging the gap between cost-effective and high-performance implants.
Global Perspective — Orthopedic Innovation Across Latin America
The success of this case in Mexico reflects a growing collaboration between Chinese orthopedic manufacturers and Latin American hospitals.
With CE and ISO 13485 certification, CZMEDITECH orthopedic implants are trusted in over 50 countries, including Mexico, Brazil, Colombia, and Chile.
Their trauma fixation systems, such as locking compression plates (LCP) and intramedullary nails, are designed to meet global clinical demands while maintaining affordable pricing for hospitals and distributors.
The Rising Demand for Orthopedic Implants in Mexico
Mexico and other Latin American regions are witnessing rapid growth in orthopedic surgeries — especially distal radius fracture fixation and wrist trauma cases.
Hospitals are increasingly choosing locking plate technology because it enables strong fixation, early rehabilitation, and reliable outcomes.
This trend demonstrates the adoption of Chinese orthopedic products in international trauma markets, reinforcing CZMEDITECH's role as a trusted partner for surgical innovation.
FAQ
Q1: What is a distal radius locking plate used for?
A1: It's designed to treat fractures at the distal end of the radius bone, ensuring angular stability and supporting early wrist movement after trauma.
Q2: Why is the VA (Variable Angle) system important?
A2: The VA system allows surgeons to adjust screw directions for optimal fixation, making it suitable for complex and osteoporotic fractures.
Q3: What makes CZMEDITECH implants reliable?
A3: Each implant is produced under CE and ISO 13485-certified quality systems, using medical-grade titanium for biocompatibility and strength.
Q4: Are CZMEDITECH orthopedic products available in Mexico and Latin America?
A4: Yes, the company supplies locking plates, trauma systems, and spine implants to hospitals across Latin America, Europe, and Asia.
Technical Highlights
| Specification | Details |
| Product | VA Distal Radius Locking Plate |
| Material | Medical-Grade Titanium Alloy |
| Features | Variable Angle Design, Biocompatible, Stable Fixation |
| Use Case | Distal Radius and Wrist Fractures |
| Certification | CE, ISO 13485 |
| Manufacturer | CZMEDITECH (China) |
| Clinical Use | Mexico, Latin America |
English
Français
Русский
Español
العربية
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
Latine
Dansk
اردو
Shqip
বাংলা
Hrvatski
Afrikaans
Gaeilge
Eesti keel
Māori
नेपाली
Oʻzbekcha
latviešu
অসমীয়া
Aymara
Azərbaycan dili
Bamanankan
Euskara
Беларуская мова
भोजपुरी
Bosanski
Български
Català
Cebuano
Corsu
ދިވެހި
डोग्रिड ने दी
Esperanto
Eʋegbe
Frysk
Galego
ქართული
guarani
ગુજરાતી
Kreyòl ayisyen
Hausa
ʻŌlelo Hawaiʻi
Hmoob
íslenska
Igbo
Ilocano
Basa Jawa
ಕನ್ನಡ
Kinyarwanda
गोंगेन हें नांव
Krio we dɛn kɔl Krio
Kurdî
Kurdî
Кыргызча
Lingala
Lietuvių
Oluganda
Lëtzebuergesch
Македонски
मैथिली
Malagasy
മലയാളം
Malti
मराठी
ꯃꯦꯇꯥꯏ (ꯃꯅꯤꯄꯨꯔꯤ) ꯴.
Mizo tawng
Chichewa
ଓଡ଼ିଆ
Afaan Oromoo
پښتو
ਪੰਜਾਬੀ
Runasimi
Gagana Samoa
संस्कृत
Gaelo Albannach
Sepeti
Sesotho
chiShona
سنڌي
Soomaali
Basa Sunda
Wikang Tagalog
Тоҷикӣ
Татарча
తెలుగు
ትግንያውያን
Xitsonga
Türkmençe
संस्कृत
ئۇيغۇرچە
Cymraeg
isiXhosa
ייִדיש
Yorùbá
isiZulu
















